Several novel mechanism-based therapeutic modalities are currently being clinically investigated for the treatment of patients with acute myelogenous leukemia (AML), including agents that exploit genomic vulnerabilities, attenuate leukemia stem cell populations and/or or synergize with anti-leukemic cytotoxic/epigenetic therapies. Lysine-specific demethylase 1 (LSD1) is an enzyme that functions in transcriptional repression by catalyzing the removal of histone H3 lysine 4 methylation, a histone modification associated with transcriptionally competent gene enhancers and transcriptional start sites. Small molecule mediated inhibition of LSD1 alters the chromatin state and the transcriptional output of LSD1 target genes. Transcriptional 'reprogramming' by LSD1 inhibitors either causes a direct impact on cell fate and/or renders malignant cells more susceptible to the treatment with other cancer therapeutic agents. LSD1 inhibitors have shown encouraging phenotypic effects in myelogenous leukemia (AML) models but the key molecular determinants governing LSD1 inhibitor sensitivity remain to be further investigated.
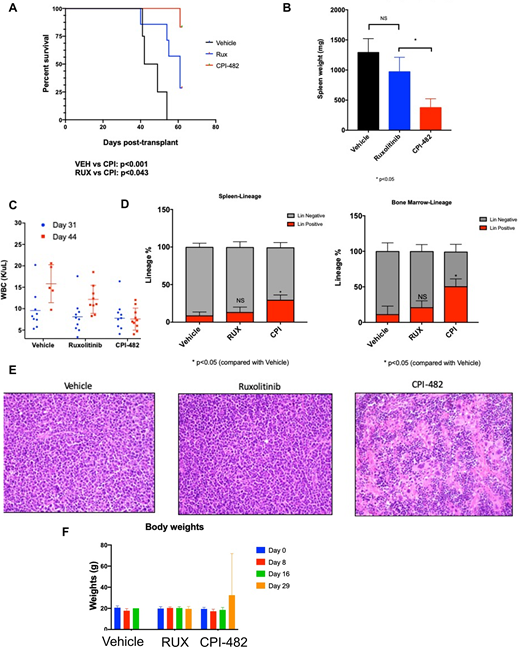
Here, we explored the in vitro sensitivity of 350 cancer cell lines to our LSD1 inhibitor CPI-482 to identify potential hyper-responder cell contexts. Four AML cell lines showed high sensitivity with low nanomolar concentration GI50s, each of which contained either a JAK2V617F mutation or a genetic aberration that resulted in JAK-STAT pathway activation. Oral administration of LSD1 inhibitor CPI-482 on a once daily or a once weekly dosing schedule resulted in significant tumor growth inhibition in SET-2 and HEL 92.1.7 JAK2 mutant AML xenograft mouse models. Given the unmet need and poor prognosis in post-MPN secondary AML (sAML) we then explored CPI-482 in a tertiary transplant post-MPN AML retroviral transduction murine model (Jak2V617F retrovirus transduced intoTp53 null bone marrow). Jak2V617F/Tp53 null spleen cells were transplanted into lethally irradiated recipient mice along with wild-type donor support whole bone marrow cells. Mice were randomized to treatment with vehicle, Ruxolitinib (60mg/kg twice daily) or CPI-482 (60mg/kg weekly). Once-weekly treatment with CPI-482 significantly improved survival compared to vehicle (p<0.001) or ruxolitinib (p<0.043) (Figure 1A). Spleen weights were significantly reduced by CPI-482 compared to ruxolitinib (p<0.05;Figure 2B). The white blood cell count was unchanged in mice treated with CPI-482 but increased in both vehicle and ruxolitinib treated mice. Evaluation at the time of terminal take-down of mice demonstrated a significant increase in the proportion of lineage positive cells in both the bone marrow and spleen (compared to vehicle, p<0.05) consistent with restoration of normal hematopoiesis (Figure 1D). Histopathologic evaluation of the spleen demonstrated marked reduction in infiltration by blast cells, restoration of lymphoid follicles, emergence of megakaryocytes (Figure 1E), and modest reductions in reticulin fibrosis in CPI-482 treated mice, which was not observed in ruxolitinib treated mice. Mice tolerated treatment with CPI-482 well, with no changes in weights of treated mice (Figure 1F).
Treatment of the JAK2V617F mutant AML cell lines SET-2 and HEL with CPI-482 resulted in specific transcriptional effects, including increased expression of the myeloid differentiation markers LY96 and CD86 and inflammatory response genes. CPI-482 also resulted in upregulation of genes that are repressed by the HOXA9 oncogene in other leukemia contexts. The induction of specific CPI-482 mediated gene expression and phenotypic changes was recapitulated by knockdown of the transcription factor GFI1B, suggesting that, consistent with prior findings in other leukemia contexts, LSD1 functionally cooperates with GFI1B in JAK2V617F mutant AML cells. These data provide support for a potential therapeutic impact of the LSD1 inhibitor CPI-482 in AML and sAML in the context of the JAK2V617F mutation, and thus extend the previous findings that LSD1 inhibitors may have utility in JAK2V617F mutated malignant proliferative neoplasms. Given the pressing need for new therapies for sAML which evolves from a pre-existing MPN, we believe these data form the rationale for a mechanism based clinical trial in this adverse risk myeloid malignancy.
Rampal:Pharmaessentia: Consultancy; Galecto: Consultancy; Abbvie: Consultancy; Stemline: Consultancy, Research Funding; Constellation: Research Funding; Incyte: Consultancy, Research Funding; Celgene: Consultancy; Promedior: Consultancy; CTI Biopharma: Consultancy; Jazz Pharmaceuticals: Consultancy; Blueprint: Consultancy. McGrath:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Xiao:Stemline Therapeutics: Research Funding. Nikom:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Wang:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Levine:Imago: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Isoplexis: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Lilly: Consultancy, Honoraria; Janssen: Consultancy; Astellas: Consultancy; Morphosys: Consultancy; Novartis: Consultancy; Amgen: Honoraria; Gilead: Honoraria; Prelude Therapeutics: Research Funding; Qiagen: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Loxo: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Trojer:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.